OUR MISSION
Physis seeks opportunities to take new and proven therapies & treatments,
and expand their geographical access through regulatory and
clinical trial facilitation.
Our mission is to be the world leader in drug development opportunities
and to furnish the most effective products at a most reasonable cost to
the patient and health care provider.

The highest ideal of cure is the speedy, gentle, and enduring restoration of health by the most trustworthy and
least harmful way.
S. Hahnemann
CONTACT US
PHYSIS-MAIL@PHYSISINTERNATIONAL.COM
TELEPHONE – (866)-611-1702
6154 TETLIN FIELD DRIVE
NEW ALBANY, OH 43054
Physis understands the role of medicine in health care, and the clinical and regulatory processes for providing therapeutic agents to those in need. Our team of experienced professionals stays current about the latest drug trends and development.
– STRATEGIC SCIENCE – STRATEGIC THERAPY –
– CLINICAL CONTROL –
– STRATEGIC COMMERCIALZATION –
THE PHYSIS APPROACH
SCIENCE MATTERS – A FOCUS ON HEALTH AND THERAPEUTIC DRUG ADAPTATION IN THE AREAS OF IMMUNE THERAPY AND PAIN
PHYSIS HAS A FOCUS ON THE IMMUNE SYSTEM, ITS ROLE IN MULTIPLE DISEASES AND THE MODULATION OF THIS SYTEM AS AN APPROACH TO THERAPY. ANCILLARY TO THIS FOCUS IS THE ROLE OF INFLAMMATION AND ITS ROLE IN DISEASE AND THE MANAGEMENT AND CONTOL OF PAIN/INFLAMMATION THROUGH THE DIRECT APPLICATION OF TRANSDERMAL THERAPEUTICS (A RISK REDUCTION APPROACH TO SYSTEMIC PAIN MODIFICATION).
CHANGING THE MICROENVIRONMENT RATHER THAN ADAPTING TO IT
EMPOWERING THERAPY BY MANAGING THE MICROENVIRONMENT – REMOVING THE GUESS WORK IN APPROACHES TO DISEASE MANAGEMENT

PHYSIS: IMMUNE MODULATION AND FUNCTIONAL CLINICAL CONTROL

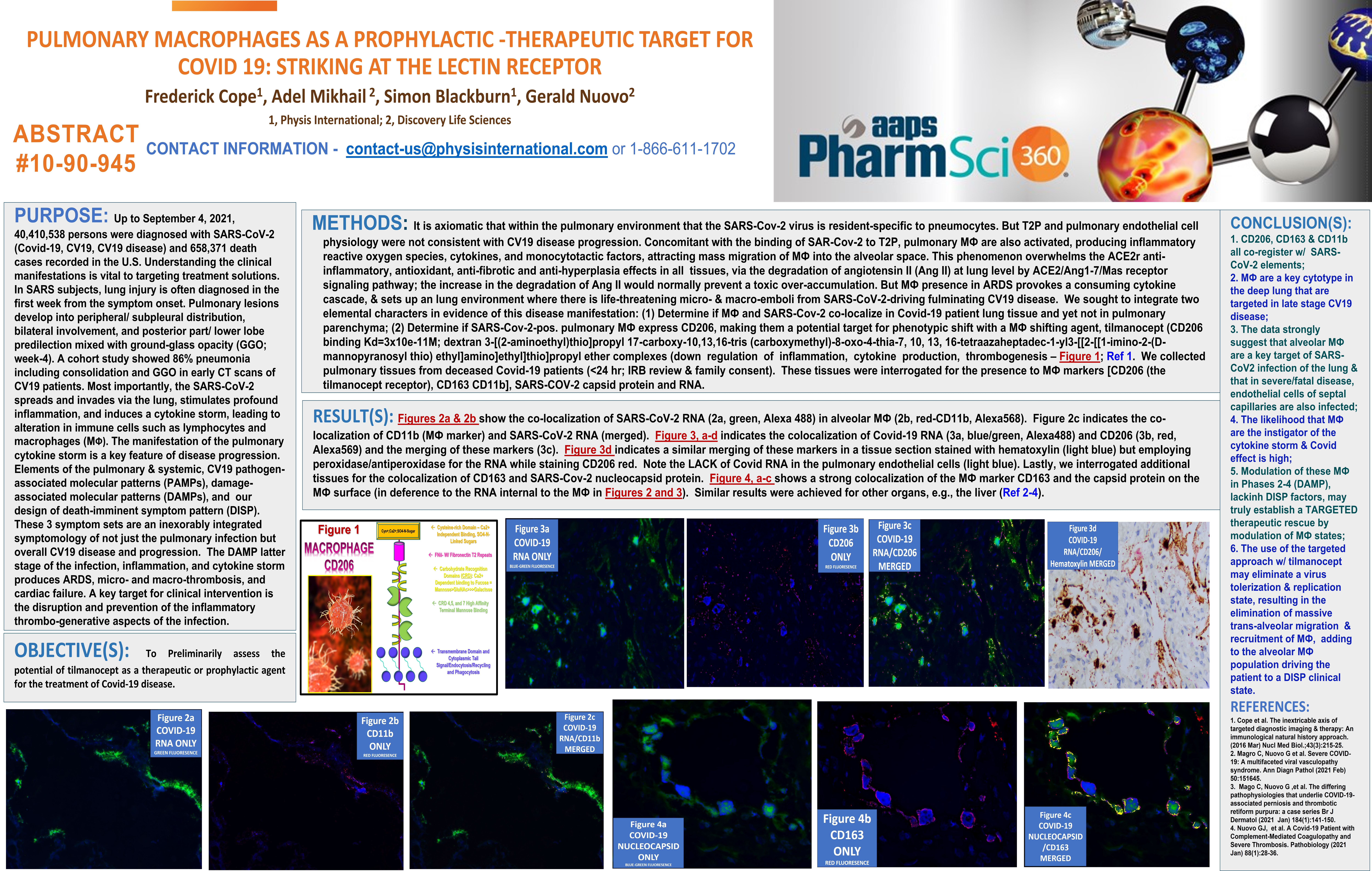
THE C-LECTIN RECEPTOR-EXPRESSING CELL
PHENOTYPE MODULATION & APOPTOSIS INDUCTION: MOLLIFYING OR KILLING THE INTENDED TARGET
A RATIONAL APPROACH TO DISEASE MODIFICATION, CLINICAL CONTROL, STRATEGIC THERAPY, EXPECTED EFFICACY, AND IMPROVED PATIENT OUTCOME
PIPELINE
SCAN TO DOWNLOAD


WHO WE ARE

Frederick O. Cope, PhD, MS, MSc(h) FACN – CEO has served as Vice President and SVP and CSO of Pharma Research & Clinical Development at Navidea from 2009 until 2018. Prior to serving at Navidea, Dr. Cope served as the Asst Director/Research, and Head of Program Research Development for The Ohio State University Comprehensive Cancer Center, The James Cancer Hospital and The Solove Research Institute. Dr. Cope also served as head of the Cancer and AIDS product development and commercialization program for the Columbus, OH Abbott Labs division, and head of human & veterinary vaccine production & improvement group for Wyeth/Ayerst Laboratories. Dr. Cope served special internships at Baylor Univ., Vanderbilt Univ., and the NCI (pathology) and as a fellow in oncology at the McArdle Laboratory for Cancer Research, the Univ. of Wisconsin-Madison and was the honored scientist in residence at the National Cancer Center Research Institute in Tokyo. He is the recipient of the Ernst W. Volwiler Award and nominee for the EROTC Marie Curie award. He has over 230 published articles, abstracts and books, and more than 40 patents. Dr. Cope has also served on the NCI SBIR review panel for more than nine years and is the recipient of more than 16 NIH grants. Dr. Cope received his B.S. from the Delaware Valley College of Science & Agriculture, his M.S. from Millersville Univ. of Pennsylvania and his Ph.D. from the Univ. of Connecticut (SCL).

Michael S, Blue, MD, FACEP, CMO/COO has served as Chief Medical Officer and medical advisor to numerous corporations including Hy-Gene Biomedical, Cardinal Health, and Navidea Biopharmaceuticals Inc, as well as technical medical consultant to investment groups. Dr. Blue has extensive clinical trials and regulatory experience in tissue engineering, medical imaging, and emergency and critical care. Dr. Blue has also served as Medical Director of Emergency Services for Mount Carmel-St Ann’s Healthcare Systems. Dr. Blue received B.S. degree from Miami of Ohio University (Phi Beta Kappa) and his M.D. degree from The Ohio State University College of Medicine (w/ Honors). He is board certified in emergency medicine and has more than 40 years’ experience in clinical medicine, research and corporate development.

Simon A. Blackburn, BA, CCRA, CCO, has more than 30 years’ experience in clinical trials, and drug and device development. Mr. Blackburn has served as project lead and head of clinical operations for numerous companies, including Cardiox Corporation, Neoprobe Corporation, Navidea Biopharma and others. Mr. Blackburn has also spearheaded Blackburn International, an international CRO in partnership with StatKing Clinical Services data management organization. Mr. Blackburn received his B.A. degree from Denison University and his CCRA examination national certification from ACRP.
ADVISORS

Brigitte Boldyreff, Dr.rer.nat.habil, B.Sc .
Dr. Boldyreff has more than 20 years’ experience in commercial biotech. She is founder of Kinase Logistics ApS, a solo company which now currently serves as European Agent for the Japanese company Carna Biosciences. She holds a PhD degree in Molecular Biology from the Univ. of Lübeck, Germany and a BS degree in Human Nutrition and Health from the Univ. College Haderslev, Denmark. She works as Executive Director at Kinase Logistics ApS, as CSO at KinaseDetect ApS and had been Assoc. Professor at the Inst. of Biochem. and Molecular Biology, Univ. of Southern Denmark. She has a strong interest in receptor and signal transduction mechanisms as these related to mechanisms of action and efficacy, especially the physiological role of kinases and their role in diseases, as well as impact of nutrition on health. In addition, she has contributed >60 peer reviewed publications in numerous areas including oncology, vascular disease, and bone diseases, among others.

Gerald Haase, MD FACS
Dr. Gerald Haase, MD, FACS, CMA, has been actively involved in medical research and clinical trials for 35 years including the design of central venous access devices, applications for intra-operative radiation therapy in pediatric solid tumors, technical surgical innovations in adult and childhood cancer. Dr. Haase also served as chairman of the Board of Scientific Counselors of the Cancer Treatment Research Fdn., and as a senior member of the Commission on Cancer of the Am. College of Surgeons. He was appointed to the Board of Trustees of the National Childhood Cancer Fdn. and served on its Medical & Scientific Advisory Board. He has published/ presented more than 200 scientific papers, is the inventor/co-inventor of 10 issued U.S. patents for micronutrient and phytonutrient therapy with 5 pending patents and been the recipient of clinical research grants/contracts funded at million-dollar cumulative levels. Dr. Haase participated with the Intl. Office of the NCI and has been an examiner in pediatric surgery for the Am. Board of Surgery. He is a member of more than 25 professional societies including the AACR, Intl. College of Surgeons, Am. Acad. of Pediatrics, Am. College of Physician Executives, & is a charter member of the Intl. Soc. of Pediatric Surgical Oncology. Dr. Haase received his undergraduate degree from the Johns Hopkins University and M.D. degree from Tufts University School of Medicine with graduate honors in research. His surgical training was at the University of Colorado, Health Sciences Center and he specialized in pediatric surgery and oncology at Children’s Hospital Medical Center, Boston, and Nationwide Children’s Hospital, Columbus, Ohio.

Olaf-Georg Issinger, Dr. rer. nat. habil.
Dr. Issinger Professor emeritus at the Department for Biochemistry and Molecular Biology at the University of Southern Denmark – Odense, DK. Dr. Issinger is founder of KinaseDetect ApS, a privately held company involved in production and sale of protein kinases, substrates and antibodies for pharmaceutical industries. Dr. Issinger’s interest in protein kinase research began as a postdoctoral fellow at the Univ. of Calif. Davis. He continued his career as Assistant professor at the Univ. of Stuttgart and Associate professor at the Medical Faculty of Saarland University – Homburg/Saar, Germany. He worked as a guest scientist at the National Cancer Center in Tokyo (1986/87) and at the St. Vincent’s Institute, Melbourne, Australia (2009). He received several special awards and serves as reviewer on various boards and international Journals. Dr. Issinger received his M.S. from the Free University in Berlin and his Ph.D. from Freiburg University, Germany. Dr. Issinger has authored over 180 scientific articles in the fields of protein kinases, signal transduction and cancer.

Adel A. Mikhail, Ph.D.
Dr. Mikhail is the founder and CEO of Phylogeny Inc., a company that specializes in tissue analyses and molecular pathology. Most recently, Dr. Mikhail has merged his company with two other organizations to form Discovery Life Sciences (Phylogeny, Conversant Bio, and Folio Bio). Dr. Mikhail has more than 25 years in bio-corporate development, investment, and biotechnology applications to new and repurposed drugs and drug development.

George Q. Mills, M.D., M.B.A.
Dr. Mills has held several senior roles at the FDA, including as a Division Director in the Center for Drugs (CDER), Branch Chief and designated Acting Deputy Division Director of the Biologics Oncology Division at the Center for Biologics Evaluation and Research (CBER). In these roles, he provided expertise in medical diagnostic imaging and therapeutic radiopharmaceutical oncologic biologics and drugs. He also served as the CBER/CDER expert in conjunction with the review of radiographic imaging submissions in support of multiple licensure submissions. Dr. Mills also served as Vice President of Medical Imaging for Perceptive Informatics, a PAREXEL Company. Dr. Mills has spoken at numerous industry conferences and has published articles in scientific journals and industry publications on imaging and regulatory topics, including the FDA Critical Path Initiative. Dr. Mills has been certified by the American Board of Nuclear Medicine and the American Board of Pathology, Anatomic and Clinical Pathology. Dr. Mills holds a M.D. Degree from Creighton University in Omaha, Nebraska, a M.B.A. degree from Pepperdine University, and a B.A. degree from the University of Nebraska.

Kenneth C. Williams, Ph.D.
Dr. Williams is Full Professor in the Department of Molecular Biology at Boston College. Dr. Williams’ work has centered on inflammation and normal and diseased states. This work includes, a) studies to define subpopulations of macrophages in the CNS and their precursors in blood and bone marrow, b) the role of the cellular immune system especially CD8 T cells controlling monocyte/macrophage activation and CNS pathogenesis, and c) developing drugs that target HIV and SIV infected/activated macrophages resulting in cell death or deactivation. In addition, we are exploring an exciting field of emergent populations of monocytes and dendritic cells that are expanded with disease. This work uses a monkey model of rapid, consistent AIDS and neuroAIDS, CD8 T lymphocyte depletion, MR spectroscopy, monocyte/macrophage biology and immunology. Dr. Williams has more than 18 years of experience in the field of inflammation and disease.